Have you ever used a can of spray suncreen and noticed how the can will get colder as you let the sunscreen out? If you missed that, perhaps you’ve noticed how cold the sunscreen can feel once it hits your skin? Both allow you to experience first-hand one of the principles of refrigeration.
A liquefied gas aerosol spray can works by having two liquids inside. The product you want out, called the payload, and the propellant. The propellant is a liquid with a low boiling point meaning that it can change from a liquid to a gas at room temperatures. When you push the button on the can, the propellant forces the product out, lowering the pressure and causing part of it to evaporate. When the propellant evaporates, it absorbs heat from its surrounding and thus the can cools off slightly. Similarly, some of the propellant will come out of the can with the product and evaporate almost instantly once it hits the air which cools the product off as well.
Refrigeration systems (kind of) do the same thing, but they also do a lot of other stuff to make it far more efficient. Refrigerators use a closed system and take advantage of the relatively large amounts of energy required to change a substance from a liquid to a gas and vice versa. Just like the propellant, refrigerants are selected based on their convenient boiling points. As you can see in the diagram below, there are 5 main “stops” on any trip around the refrigeration cycle.
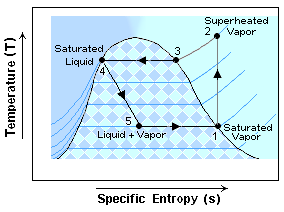
It’s always tough to know where to start, so we’ll start with number 1. At stop 1, or the entrance to the compressor, the refrigerant is what is called a saturated vapor. This means that every bit of the refrigerant is vapor and any additional energy added at this point would only make it a superheated vapor which is exactly what the compressors do between stops 1 and 2. At stop 2, we exit the compressor and enter the condenser where the refrigerant starts to cool down. In the condenser, the refrigerant changes from a superheated vapor back to a saturated vapor (stop 3), then to a mixture (part vapor and part liquid) and finally end up at 100% liquid which is also called a saturated liquid (stop 4). From there the liquid refrigerant goes through a valve which lowers the pressure allowing the liquid to flash into a gas at a much colder temperature. Now that we are at stop 5 and the refrigerant is cold, it starts absorbing heat from its surroundings which is what produces the cooling effect (remember that can of suncreen?). Once it has absorbed all the energy it efficiently can, it’s back to the compressors at stop 1.
There’s a lot more that can be added to a refrigeration system to increase it’s efficiency, but that will have to wait for a future article. At Forward Engineers, we seek to not only be a design and consulting firm but to also educate our clients about engineering technology. While doing so, we inevitably refresh our own knowledge and sometimes even learn something ourselves. If you are seeking to work with an engineering firm that is client-centered and strives to provides services that are on time, on budget and exceed expectations, please contact us. We would love to work with you on your next project!



